MBQ-167 efficacy
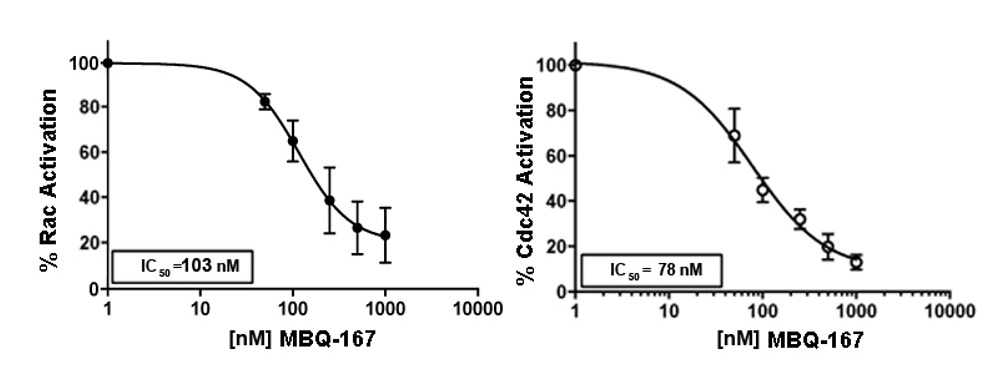
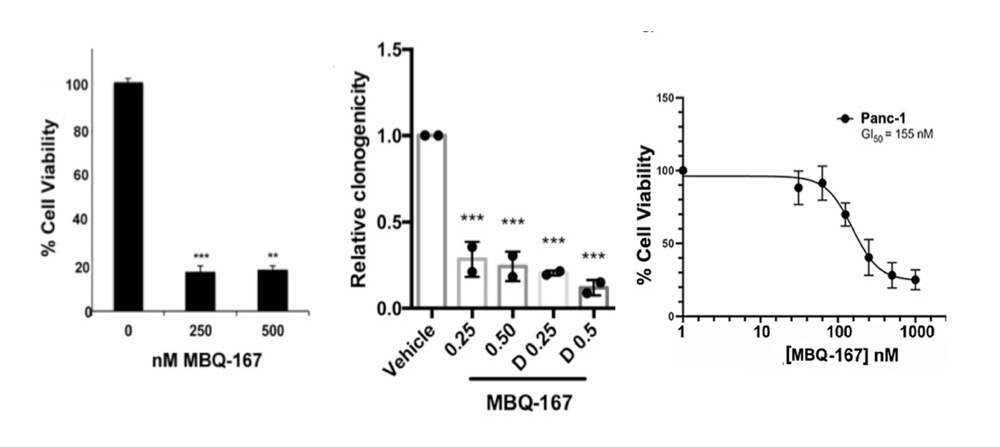
MBQ-167 targets the GTPases Rac1 and Cdc42 simultaneously. Rac1 and CDC42 are novel therapeutic targets which control the activity of the cancer cell skeleton, a critical functional element connected to the process of metastasis and tumor cell growth. MBQ-167 is a first-in-class, selective GTPase inhibitor which has been shown to prevent metastasis and remove pre-existing metastases for breast cancer tumors in various mouse models.
MBQ-167 safety
Preclinical development of MBQ-167 revealed an excellent safety profile and good bioavailability and pharmacokinetics. Based on these encouraging data for an excellent safety profile, the USFDA accepted in June 2022 the Investigational New Drug (IND) for a Phase 1 Open-Label, First-in-Human Trial of Oral MBQ-167 as Single Agent in Subjects with Advanced Breast Cancer.
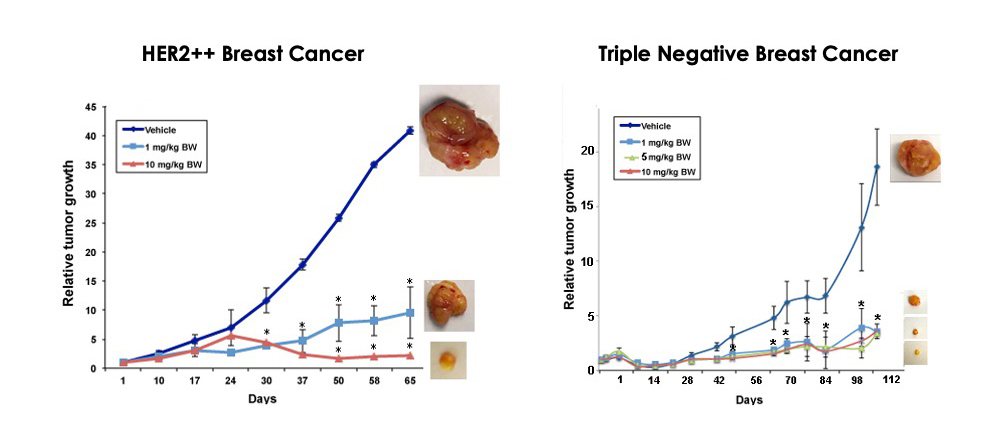
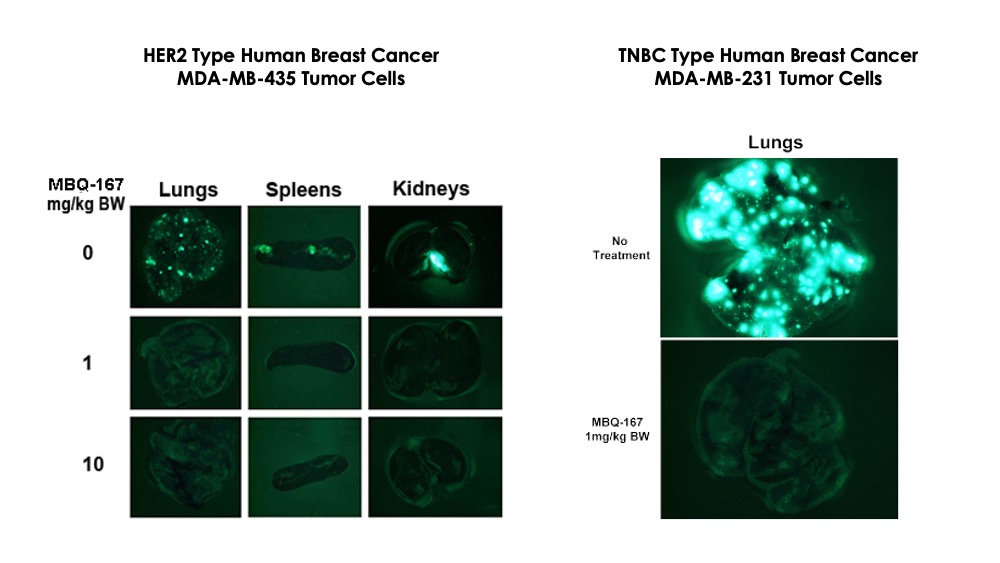
MBQ-167 monotherapy inhibits breast cancer metastasis
MBQ-167 not only reduces primary tumor growth rates, but also prevents metastasis. The experiment below demonstrates both reduction in tumor growth rates for two different cancer cell lines as well as prevention of metastases. MBQ-167 inhibits intravasation (spread of breast cancer from breast tumors (human breast cancer cells tagged with green fluorescent protein) to distant organs and extravasation (direct colonization of distant organs, such as the lung).
Experimental Design
Day 0:
Inoculate GFP-tagged MDA-MB-435 or MDA-MB-231 cells into mammary fat pads in SCID mice
Day 7:
Administer MBQ-167 intraperitoneally when the tumor size reaches about 100 mm3
Day 60:
Image tumor fluorescence
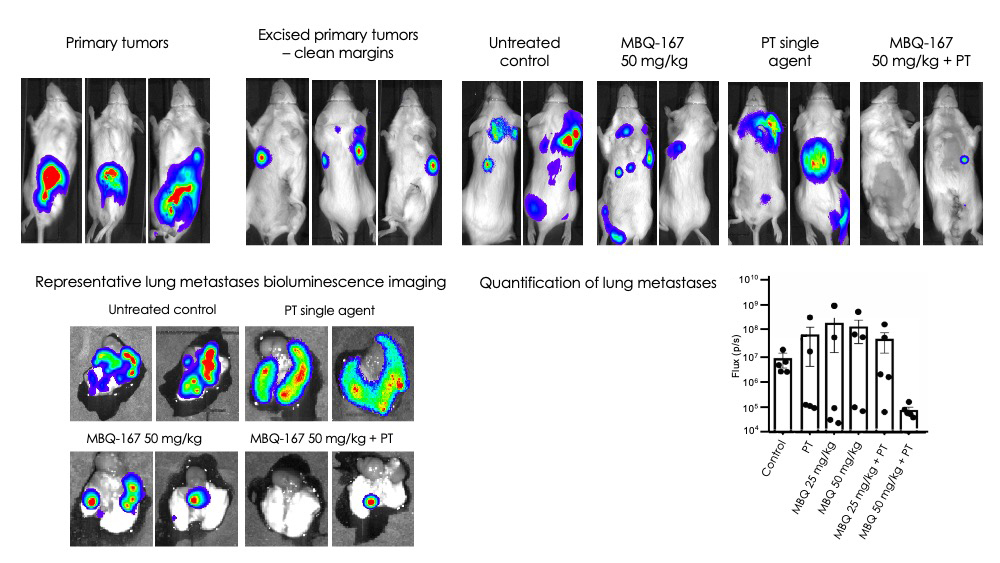
MBQ-167 synergizes with paclitaxel to remove established metastases
In addition to reduction in primary tumor growth rates and prevention of metastases, MBQ-167 together with a standard of care such as paclitaxel removes pre-existing metastases. This can be shown with a study design such as the one in the experiment below. A key step in this experiment is the removal of the primary 4T1 cell tumors before treatment, to make sure that the combined MBQ-167/Paclitaxel therapy removes pre-existing metastases, independently of MBQ-167 inhibition of primary tumor growth.
Experimental Design
Day 0
Establish 4T1 cell tumors
Day 14
Excise primary tumors
Day 21
Image lungs and quantify metastasis base level
Day 21 – Day 38
Oral treatment with MBQ-167 5 times a week
Day 38
Image and excise lungs and quantify metastasis
No MBQ-167-dependent toxicity in standard IND nonclinical safety assessment package
MBQ-167 nonclinical safety was evaluated throughout the UPR R&D period of 2010-2017 and the MBQ Pharma MBQ-167 nonclinical development from 2018-2022. The MBQ-167 GLP nonclinical safety and safety pharmacology assessment packages show a NOAEL in rats and dogs of 1,000 mg/kg/day, and no MBQ-167-dependent findings in the FDA-required tests. The therapeutic dose window for MBQ-167 in these animal models is quite broad, with a minimum therapeutic dose of approximately 100 mg/kg/day.
| 2010 – 2017 | 2018 – 2021 |
|---|---|
| Initial in vitro and in vivo studies as part of sponsored academic research projects: No findings |
|
2010 – 2017:
Initial in vitro and in vivo studies as part of sponsored academic research projects: No findings
2018 – 2021:
- Ames Test: Negative
- In vivo Micronucleus Assay: Negative
- Non-GLP rat dose escalation and repeat dose studies: Negative whole animal, pathology reports and phagocytosis assay. (NOAEL = 1,000 mg/kg)
- Non-GLP dog dose escalation studies: Negative whole animal and pathology reports (NOAEL = 1,000 mg/kg)
- GLP 28-day rat and dog repeat dose studies: Negative whole animal and pathology reports
- GLP Respiratory, CNS, Cardiovascular, hERG safety pharmacology studies: Negative
- In vitro 3T3 neutral red uptake phototoxicity assay: Positive (avoid sun exposure)
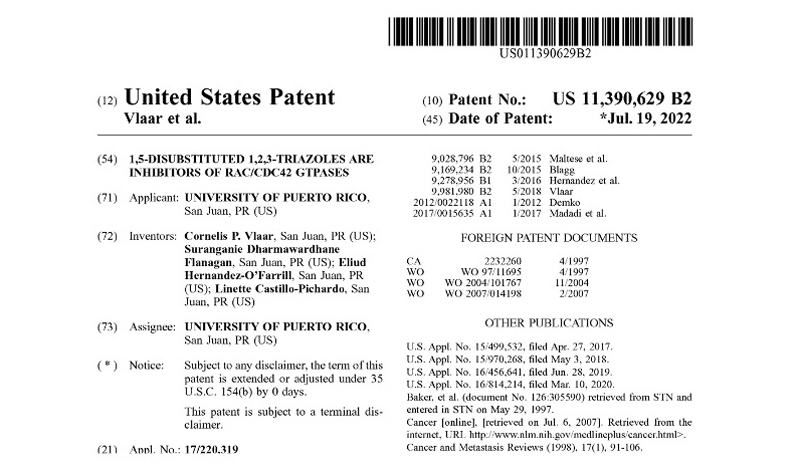
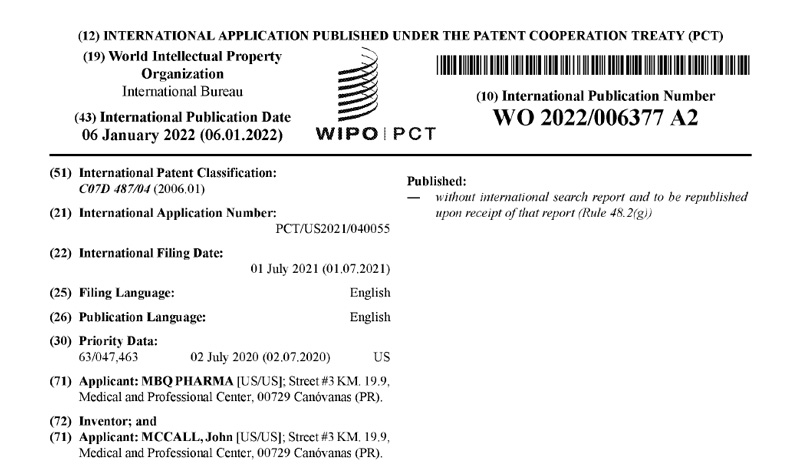
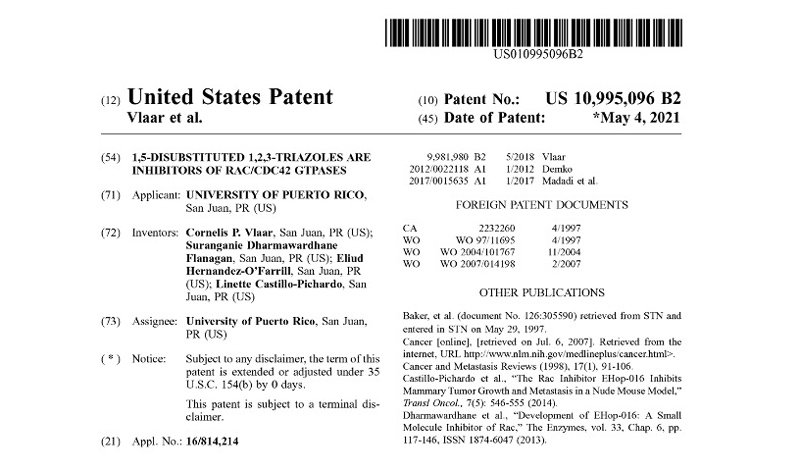
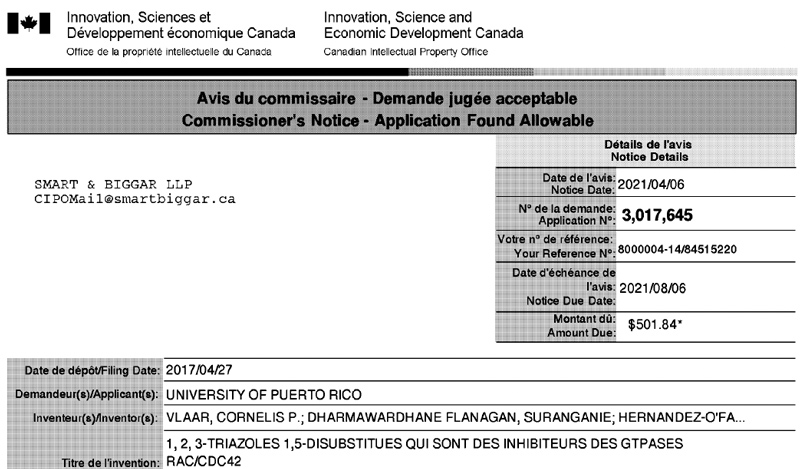
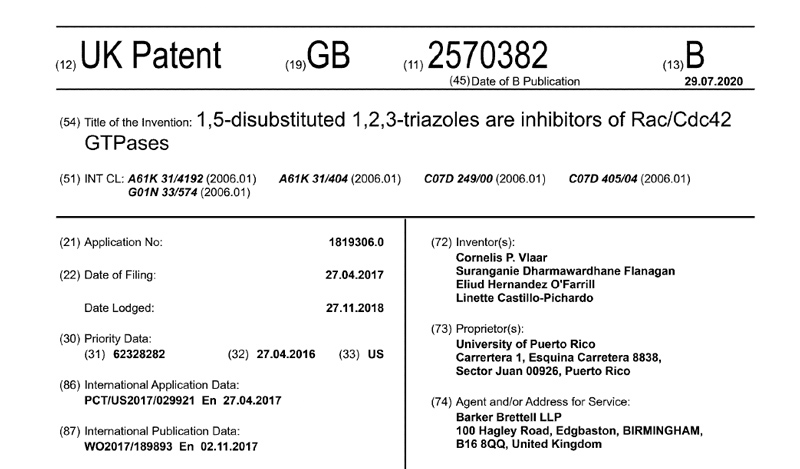
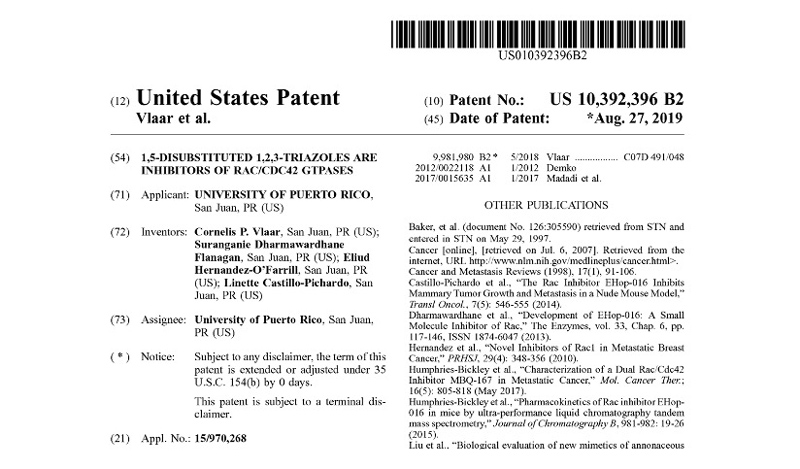
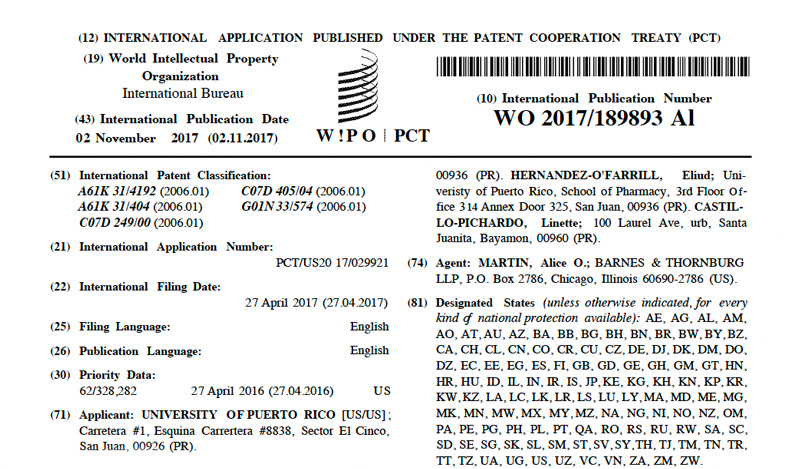
Patents under License or Assigned to MBQ Pharma
References
- Medina JI, Cruz-Collazo A, Del Mar Maldonado M, Gascot TM, Borrero-Garcia LD, Cooke M, Kazanietz MG, O’Farril EH, Vlaar CP, Dharmawardhane S. Characterization of Novel Derivatives of MBQ-167, an inhibitor of the GTP-binding proteins Rac/Cdc42. Cancer Res Commun. 2022 Dec;2(12):1711-1726. doi: 10.1158/2767-9764.crc-22-0303. Epub 2022 Dec 29. PMID: 36861094; PMCID: PMC9970268.
- Cruz-Collazo A, Ruiz-Calderon JF, Picon H, Borrero-Garcia LD, Lopez I, Castillo-Pichardo L, Del Mar Maldonado M, Duconge J, Medina JI, Bayro MJ, Hernández-O’Farrill E, Vlaar CP, Dharmawardhane S. Efficacy of Rac and Cdc42 Inhibitor MBQ-167 in Triple-negative Breast Cancer. Mol Cancer Ther. 2021 Dec;20(12):2420-2432. doi: 10.1158/1535-7163.MCT-21-0348. Epub 2021 Oct 4. PMID: 34607932; PMCID: PMC8643341.
- Jiménez Cruz JM, Vlaar CP, Stelzer T, López-Mejías V. Polymorphism in early development: The account of MBQ-167. Int J Pharm. 2021 Oct 25;608:121064. doi: 10.1016/j.ijpharm.2021.121064. Epub 2021 Sep 1. PMID: 34481010; PMCID: PMC9675584.
- Jiménez Cruz JM, Vlaar CP, López-Mejías V, Stelzer T. Solubility Measurements and Correlation of MBQ-167 in Neat and Binary Solvent Mixtures. J Chem Eng Data. 2021 Jan 14;66(1):832-839. doi: 10.1021/acs.jced.0c00908. Epub 2020 Dec 10. PMID: 36262318; PMCID: PMC9578765.
- Borrero-García LD, Del Mar Maldonado M, Medina-Velázquez J, Troche-Torres AL, Velazquez L, Grafals-Ruiz N, Dharmawardhane S. Rac inhibition as a novel therapeutic strategy for EGFR/HER2 targeted therapy resistant breast cancer. BMC Cancer. 2021 Jun 1;21(1):652. doi: 10.1186/s12885-021-08366-7. PMID: 34074257; PMCID: PMC8170972.
- Dharmawardhane S. Rho Family GTPases in Cancer. Cancers (Basel). 2021 Mar 12;13(6):1271. doi: 10.3390/cancers13061271. PMID: 33809395; PMCID: PMC7998900.
- Reig-López J, Maldonado MDM, Merino-Sanjuan M, Cruz-Collazo AM, Ruiz-Calderón JF, Mangas-Sanjuán V, Dharmawardhane S, Duconge J. Physiologically-Based Pharmacokinetic/Pharmacodynamic Model of MBQ-167 to Predict Tumor Growth Inhibition in Mice. Pharmaceutics. 2020 Oct 15;12(10):975. doi: 10.3390/pharmaceutics12100975. PMID: 33076517; PMCID: PMC7602742.
- Maldonado MDM, Medina JI, Velazquez L, Dharmawardhane S. Targeting Rac and Cdc42 GEFs in Metastatic Cancer. Front Cell Dev Biol. 2020 Apr 8;8:201. doi: 10.3389/fcell.2020.00201. PMID: 32322580; PMCID: PMC7156542.
- Maldonado MDM, Rosado-González G, Bloom J, Duconge J, Ruiz-Calderón JF, Hernández-O’Farrill E, Vlaar C, Rodríguez-Orengo JF, Dharmawardhane S. Pharmacokinetics of the Rac/Cdc42 Inhibitor MBQ-167 in Mice by Supercritical Fluid Chromatography-Tandem Mass Spectrometry. ACS Omega. 2019 Oct 23;4(19):17981-17989. doi: 10.1021/acsomega.9b01641. PMID: 31720502; PMCID: PMC6843717.
- Rivera-Robles MJ, Medina-Velázquez J, Asencio-Torres GM, González-Crespo S, Rymond BC, Rodríguez-Medina J, Dharmawardhane S. Targeting Cdc42 with the anticancer compound MBQ-167 inhibits cell polarity and growth in the budding yeast S. cerevisiae. Small GTPases. 2020 Nov;11(6):430-440. doi: 10.1080/21541248.2018.1495008. Epub 2018 Jul 29. PMID: 29969362; PMCID: PMC7549613.
- Maldonado MDM, Dharmawardhane S. Targeting Rac and Cdc42 GTPases in Cancer. Cancer Res. 2018 Jun 15;78(12):3101-3111. doi: 10.1158/0008-5472.CAN-18-0619. Epub 2018 Jun 1. PMID: 29858187; PMCID: PMC6004249.
- Vlaar CP, Castillo-Pichardo L, Medina JI, Marrero-Serra CM, Vélez E, Ramos Z, Hernández E. Design, synthesis and biological evaluation of new carbazole derivatives as anti-cancer and anti-migratory agents. Bioorg Med Chem. 2018 Feb 15;26(4):884-890. doi: 10.1016/j.bmc.2018.01.003. Epub 2018 Jan 11. PMID: 29358027; PMCID: PMC5822041.
- Humphries-Bickley T, Castillo-Pichardo L, Hernandez-O’Farrill E, Borrero-Garcia LD, Forestier-Roman I, Gerena Y, Blanco M, Rivera-Robles MJ, Rodriguez-Medina JR, Cubano LA, Vlaar CP, Dharmawardhane S. Characterization of a Dual Rac/Cdc42 Inhibitor MBQ-167 in Metastatic Cancer. Mol Cancer Ther. 2017 May;16(5):805-818. doi: 10.1158/1535-7163.MCT-16-0442. PMID: 28450422; PMCID: PMC5418092.
- Veluthakal R, Tunduguru R, Arora DK, Sidarala V, Syeda K, Vlaar CP, Thurmond DC, Kowluru A. VAV2, a guanine nucleotide exchange factor for Rac1, regulates glucose-stimulated insulin secretion in pancreatic beta cells. Diabetologia. 2015 Nov;58(11):2573-81. doi: 10.1007/s00125-015-3707-4. Epub 2015 Jul 31. PMID: 26224100; PMCID: PMC4591202.
- Sidarala V, Veluthakal R, Syeda K, Vlaar C, Newsholme P, Kowluru A. Phagocyte-like NADPH oxidase (Nox2) promotes activation of p38MAPK in pancreatic β-cells under glucotoxic conditions: Evidence for a requisite role of Ras-related C3 botulinum toxin substrate 1 (Rac1). Biochem Pharmacol. 2015 Jun 15;95(4):301-10. doi: 10.1016/j.bcp.2015.04.001. Epub 2015 Apr 14. PMID: 25881746; PMCID: PMC6684092.
- Humphries-Bickley T, Castillo-Pichardo L, Corujo-Carro F, Duconge J, Hernandez-O’Farrill E, Vlaar C, Rodriguez-Orengo JF, Cubano L, Dharmawardhane S. Pharmacokinetics of Rac inhibitor EHop-016 in mice by ultra-performance liquid chromatography tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2015 Feb 15;981-982:19-26. doi: 10.1016/j.jchromb.2014.12.021. Epub 2015 Jan 2. PMID: 25594952; PMCID: PMC4306626.
- Castillo-Pichardo L, Humphries-Bickley T, De La Parra C, Forestier-Roman I, Martinez-Ferrer M, Hernandez E, Vlaar C, Ferrer-Acosta Y, Washington AV, Cubano LA, Rodriguez-Orengo J, Dharmawardhane S. The Rac Inhibitor EHop-016 Inhibits Mammary Tumor Growth and Metastasis in a Nude Mouse Model. Transl Oncol. 2014 Oct 24;7(5):546-55. doi: 10.1016/j.tranon.2014.07.004. PMID: 25389450; PMCID: PMC4225654.
- Martin H, Mali RS, Ma P, Chatterjee A, Ramdas B, Sims E, Munugalavadla V, Ghosh J, Mattingly RR, Visconte V, Tiu RV, Vlaar CP, Dharmawardhane S, Kapur R. Pak and Rac GTPases promote oncogenic KIT-induced neoplasms. J Clin Invest. 2013 Oct;123(10):4449-63. doi: 10.1172/JCI67509. Epub 2013 Sep 16. PMID: 24091327; PMCID: PMC3784531.
- Dharmawardhane S, Hernandez E, Vlaar C. Development of EHop-016: a small molecule inhibitor of Rac. Enzymes. 2013;33 Pt A(Pt A):117-46. doi: 10.1016/B978-0-12-416749-0.00006-3. Epub 2013 Aug 8. PMID: 25033803; PMCID: PMC6512336.
- Montalvo-Ortiz BL, Castillo-Pichardo L, Hernández E, Humphries-Bickley T, De la Mota-Peynado A, Cubano LA, Vlaar CP, Dharmawardhane S. Characterization of EHop-016, novel small molecule inhibitor of Rac GTPase. J Biol Chem. 2012 Apr 13;287(16):13228-38. doi: 10.1074/jbc.M111.334524. Epub 2012 Mar 1. PMID: 22383527; PMCID: PMC3339933.
- Hernández E, De La Mota-Peynado A, Dharmawardhane S, Vlaar CP. Novel inhibitors of Rac1 in metastatic breast cancer. P R Health Sci J. 2010 Dec;29(4):348-56. PMID: 21261173.
- Azios NG, Krishnamoorthy L, Harris M, Cubano LA, Cammer M, Dharmawardhane SF. Estrogen and resveratrol regulate Rac and Cdc42 signaling to the actin cytoskeleton of metastatic breast cancer cells. Neoplasia. 2007 Feb;9(2):147-58. doi: 10.1593/neo.06778. PMID: 17356711; PMCID: PMC1813930.
- Baugher PJ, Krishnamoorthy L, Price JE, Dharmawardhane SF. Rac1 and Rac3 isoform activation is involved in the invasive and metastatic phenotype of human breast cancer cells. Breast Cancer Res. 2005;7(6):R965-74. doi: 10.1186/bcr1329. Epub 2005 Sep 30. PMID: 16280046; PMCID: PMC1410764.